Drug Delivery Platform Manipulating Amyloid Proteins Key to Alzheimer’s Treatment
“Understanding the structural and assembly properties of the beta-amyloid 42 peptide is crucial for advancing our knowledge of Alzheimer’s and developing targeted therapies for this devastating neurodegenerative disorder,” said Ronit Freeman, senior author of the study, “Uncovering Supramolecular Chirality Codes for the Design of Tunable Biomaterials,” and an associate professor in the Department of Applied Physical Sciences (APS).
APS scientists partnered with the Lynn lab at Emory University to examine the key portion of the beta-amyloid 42 peptide driving plaque assembly and deposits in the brains of patients with Alzheimer’s disease. By creating synthetic variations of the peptide in the lab, the researchers, using advanced spectroscopic techniques, were able to discover how to control the way that these molecules assemble and twist either in a right-handed (clockwise) or left-handed (counterclockwise) direction.
The researchers further showed that a temperature-triggered inversion or reversal of the direction of the helical structure of amyloid fibrils can release drugs loaded or attached to the fibrils.
“Helical chirality, with left- or right-handed supramolecular twists, is a common motif in living systems with implications for both normal function and dysfunction,” said Freeman. “In a disease like Alzheimer’s, the predominant helical chirality of amyloid assemblies may impact disease progression and accumulation.”
Freeman said the ability of these amyloid materials to untwist and degrade highlights potential for treatments modifying and subsequently reversing plaques found in Alzheimer’s and other neurodegenerative diseases.
“We know that the direction of the amyloid fibril twists is associated with different disease progression states,” she said. “Imagine that by a simple treatment, we could modify amyloids to change their shape and disappear—this is what our discovery might enable us to do in the future.”
To learn more, visit the Freeman Lab for advances and scientific discovery.
 Ronit Freeman, senior author of the study and an APS associate professor
Ronit Freeman, senior author of the study and an APS associate professor
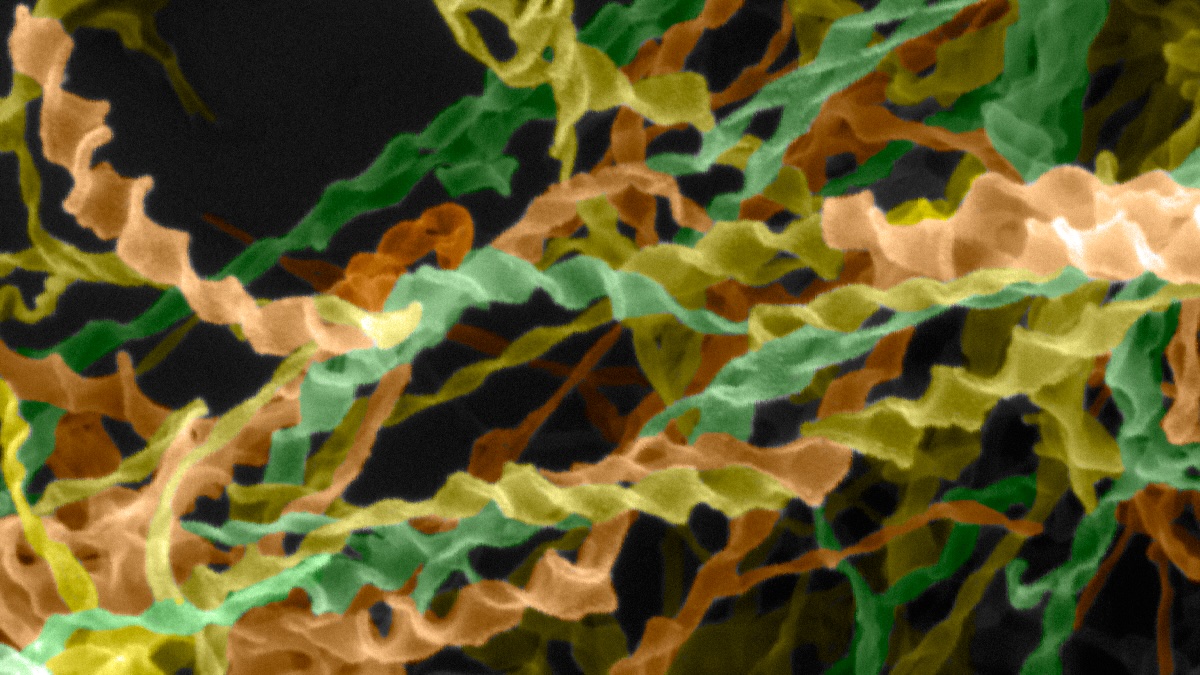 An electron microscopy image (falsely colored) of a beta-amyloid-derived peptide exhibiting helical twists.
An electron microscopy image (falsely colored) of a beta-amyloid-derived peptide exhibiting helical twists.

