Behavior of Well-Known Protein Provides Clues to How Cells Sense Their Shape
Researchers have developed a model for how specialized proteins called septins can bind to and organize on cell membranes, according to a paper co-authored by an Applied Physical Sciences professor and published in the Proceedings of the National Academy of Sciences.
The research has implications for the treatment of disease, since malfunctions in septins—that is, an inability to sense and respond to changes in a cell membrane’s curvature—have been linked to clinical neurodevelopmental disorders, hemophilia, cancer and infertility.
“The findings contribute to our understanding of cellular processes and may have broader applications in cell biology and biophysics,” said Assistant Professor Ehssan Nazockdast, who led the research that appears in the peer-reviewed article, “Curvature Sensing as an Emergent Property of Multiscale Assembly of Septins.”
Nazockdast collaborated on the research with Amy Gladfelter, a professor at the Duke University School of Medicine, and Wenzheng Shi, a Ph.D. student in Applied Physical Sciences and the article’s first author.
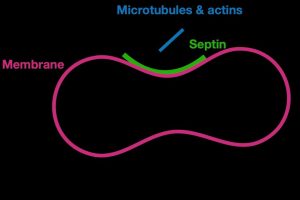
For a cell to perform many of its functions, it first needs to sense the shape of its exterior boundary, or membrane, which acts as a traffic cop mediating interactions between its inside and outside environments. Septins form long filaments and act as scaffolds, recruiting other proteins into their assigned role of builders of cell infrastructure. Nazockdast said their model provides a quantitative framework for understanding and predicting the behavior of septin assemblies, or structures, which are important to maintaining cell shape, cell division and other cellular processes.
“The paper shows that, unlike most proteins that sense curvature in single protein level, septins sense curvature by polymerizing and assembling septin filaments into much larger micron-scale structures,” he said. “This is very interesting because it means, depending on the process and the timing of it, the cell can tune the preferred curvature of septins by changing the parameters that control its assembly.”
Rod-like septins sense a cell membrane’s barbell-like curvature through a series of complex chemical reactions that are involved in regulating cell activity. Septin assembly on curved membranes, they said, appears to be a series of steps in which smaller units of septins bind to a cell membrane, then attract other proteins to create a scaffold of longer, more stable filaments. The findings also suggest that septins may control their curvature preference by modulating their length, which could be a complex process involving multiple factors.
“This multistep hierarchical assembly on the membrane is the key to their curvature-sensing ability,” said Nazockdast. “This mechanism allows septins to be adaptable and versatile in different cellular contexts.”
Nazockdast said understanding the mechanism through which septins sense shape may also find applications in synthetic biology, a multidisciplinary field of science focusing on living systems and organisms that applies engineering principles in developing biological parts, devices and systems or to redesign existing systems found in nature.
“The interplay between nanoscale proteins and their effect on the shape of cells is a profound mystery that continues to unravel, and the septin proteins provide a captivating glimpse into this intricate dance,” said Nazockdast. “As we delve deeper into the mechanics of septin assembly, we inch closer to a comprehensive understanding of how cells perceive and communicate their shape, a fundamental question that has intrigued biologists for generations.”
APS Assistant Professor Ehssan Nazockdast, left, collaborated on the research with Wenzheng Shi, an APS Ph.D. student and the article’s first author.

